AI Accelerates Discovery of Targeted Antibiotic for Gut Infections
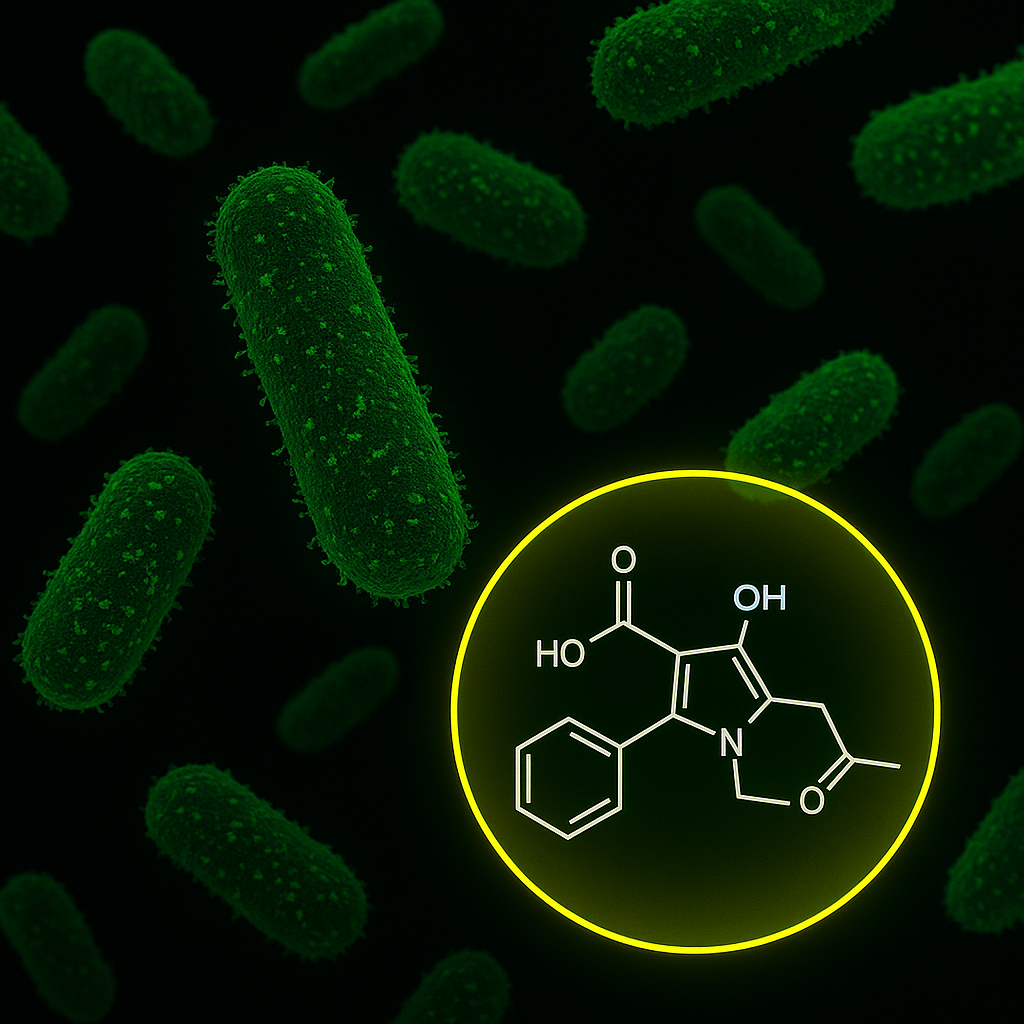
A collaboration between MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) and McMaster University has led to the rapid identification of a promising new antibiotic compound, enterololin, which targets harmful gut bacteria linked to Crohn’s disease while sparing beneficial microbes. Using a generative AI model called DiffDock, the researchers mapped the compound’s mechanism of action in just months—a process that traditionally takes years. This breakthrough offers hope for patients with inflammatory bowel disease (IBD), who often face the unintended consequences of broad-spectrum antibiotics.
Enterololin represents a step forward in precision medicine. In mouse models of Crohn’s-like inflammation, the compound selectively attacked Escherichia coli, a bacterium known to exacerbate gut flare-ups, while preserving the rest of the microbiome. Mice treated with enterololin recovered more quickly and maintained healthier microbial diversity compared to those given vancomycin. “This discovery speaks to a central challenge in antibiotic development,” said Jon Stokes, senior author of the study and research affiliate at MIT’s Abdul Latif Jameel Clinic. “The problem isn’t finding molecules that kill bacteria in a dish… A major hurdle is figuring out what those molecules actually do inside bacteria.”
To overcome that hurdle, the team turned to DiffDock, an AI model developed by MIT researchers Gabriele Corso and Regina Barzilay. Unlike traditional docking algorithms, DiffDock uses probabilistic reasoning to predict how small molecules bind to proteins. Within minutes, it identified LolCDE—a protein complex essential for lipoprotein transport—as enterololin’s target. Experimental validation confirmed the prediction, with resistant E. coli mutants and CRISPR knockdowns pointing to disruptions in the same pathway. “When you see the computational model and the wet-lab data pointing to the same mechanism, that’s when you start to believe you’ve figured something out,” Stokes noted.
The implications extend beyond this single compound. Narrow-spectrum antibiotics have long been sought for their ability to treat infections without damaging the microbiome, but they’ve been difficult to develop. By dramatically shortening the timeline for mechanism-of-action studies, AI tools like DiffDock could unlock a new generation of targeted therapies. Stokes’ spinout company, Stoked Bio, has licensed enterololin and is optimizing it for human use, with early work exploring its potential against other resistant pathogens. As Barzilay emphasized, “AI can also provide mechanistic explanations, which are critical for moving a molecule through the development pipeline.”